Every year, the FDA issues hundreds of safety alerts about medicines, medical devices, food, and other regulated products. Some of these alerts warn about deadly side effects. Others reveal hidden defects in devices used in hospitals. A few even tell you to stop using a product immediately. If you’re someone who relies on insulin, uses a pacemaker, or cares for an elderly parent on multiple medications, missing one of these alerts could put your health-or someone else’s-at risk.
What Are FDA Safety Communications?
FDA Safety Communications are official, real-time updates issued by the U.S. Food and Drug Administration when new safety risks are discovered after a product has been approved and is already on the market. These aren’t press releases or vague warnings. They’re specific, actionable notices that tell you exactly what’s wrong, which products are affected, and what you should do next.
There are three main types:
- Medical Device Safety Communications - These cover problems with things like insulin pumps, heart monitors, joint replacements, and even pregnancy tests. In 2022 alone, the FDA issued 45 of these for devices.
- Drug Safety Communications - These warn about dangerous side effects in prescription and over-the-counter medicines. Think blood thinners that interact badly with new supplements, or diabetes drugs linked to rare kidney issues.
- Enforcement Reports - These are recall notices. When a company is ordered to pull a product off shelves because it’s unsafe, contaminated, or mislabeled, this is where you’ll find it.
Before 2021, these alerts were scattered across different pages on FDA.gov. You had to check manually. Now, you can get them delivered straight to your inbox-no searching needed.
How the Subscription System Works
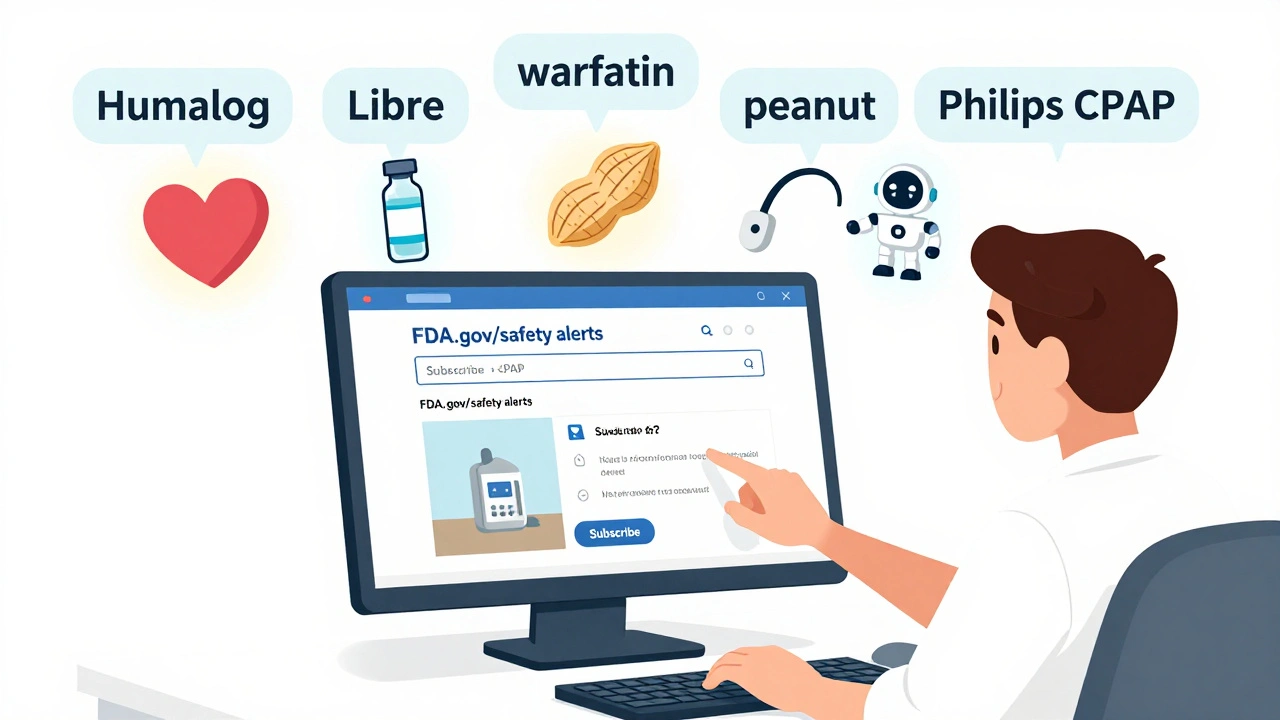
The FDA’s Enforcement Report Subscription Service, updated in July 2022, lets you pick up to five keywords that matter to you. You don’t get every alert. You get only the ones tied to your interests.
For example:
- If you have a peanut allergy, subscribe to peanut. You’ll get notified if any snack, medicine, or supplement is recalled for undeclared peanut ingredients.
- If you use a continuous glucose monitor, subscribe to glucose monitor or the brand name like Libre or Guardian.
- If you’re a caregiver for someone on blood thinners, subscribe to warfarin or apixaban.
It’s simple. Go to FDA.gov/safety/alerts, click "Subscribe," enter your email, and type in your keywords. That’s it.
But here’s the catch: you have to know what to search for. If you’re not sure what your device is called, check the label or the manual. If you’re unsure about a medication’s generic name, ask your pharmacist. The system won’t guess for you-it only sends alerts based on exact keyword matches.
Early Alerts: The Hidden Safety Net
On September 29, 2025, the FDA expanded its Early Alert Communications Program to cover all medical devices. Before that, it only covered a few categories like heart and kidney devices. Now, it includes everything-from hearing aids to surgical robots.
Early Alerts are different from recalls. They’re not official recalls yet. But the FDA has enough evidence to believe a device might be dangerous. Maybe there’s a pattern of malfunctions. Maybe a few patients had serious injuries. The agency doesn’t wait for a full investigation before warning people.
These alerts include:
- What product is affected
- Why there’s concern
- What you should do right now (e.g., "stop using," "contact your doctor," "check for firmware updates")
- Any reported deaths or injuries linked to the device
This is the closest thing the FDA has to a real-time early warning system. It’s designed to act before people get hurt-not after.
Why This Matters for Patients and Caregivers
Imagine you’re using a ventilator at home for a loved one with COPD. One day, the machine starts making a strange noise. You call the manufacturer. They say it’s normal. But you don’t know that the FDA issued an Early Alert two days ago about that exact model failing during high-pressure use-resulting in three hospitalizations.
If you’re not subscribed, you never hear about it. You keep using it. And then something goes wrong.
That’s why this isn’t just about staying informed. It’s about survival. The FDA doesn’t just monitor safety. It acts on real-world data from over 300 million patient records through its Sentinel System. These alerts aren’t guesses. They’re based on actual harm patterns.
Parents of children with asthma should watch for alerts on inhalers. Seniors on blood pressure meds should track warnings about drug interactions. Even people taking OTC painkillers need to know if a batch is contaminated.
What Manufacturers and Providers Need to Know
If you work in healthcare or run a medical device company, ignoring these alerts isn’t just risky-it’s legally dangerous.
Manufacturers are expected to monitor FDA communications as part of their quality systems. If a device you sell gets flagged, and you don’t update your warnings or recall instructions, you could face fines, lawsuits, or even criminal charges.
For doctors and nurses, these alerts help guide clinical decisions. A 2023 study in JAMA Internal Medicine found that providers who regularly checked FDA safety updates were more likely to catch adverse events early and adjust treatment plans before harm occurred.
These aren’t optional reading. They’re part of professional responsibility.
How to Set It Up Right
Here’s how to make sure you’re getting the right alerts:
- Go to FDA.gov/safety/alerts.
- Click "Subscribe to Email Alerts" under "Enforcement Reports."
- Enter your email address.
- Under "Keywords," type up to five terms. Use specific names: "Humalog," not "insulin." "Philips CPAP," not "sleep apnea machine."
- Check "Medical Device Safety and Recalls" separately if you want device-specific alerts.
- Confirm your subscription via the email you receive.
Pro tip: If you’re unsure what to search for, look at the labels on your devices or medications. The generic name is often listed right under the brand name. Write those down and use them as keywords.
You’ll start getting emails within hours of new alerts. The frequency depends on your keywords. Someone tracking "peanut" might get one alert a year. Someone tracking "insulin pump" might get three in a month.
What You Won’t Get
Don’t expect these alerts to tell you:
- Which pharmacies have the recalled product
- How to get a replacement
- Compensation options
The FDA’s job is to warn you. It’s up to you (or your provider) to take the next step. For recalls, the manufacturer usually handles replacements. For drug interactions, your pharmacist can help. For device issues, contact the company directly.
But without the alert? You won’t even know to ask.
What’s Next for FDA Alerts?
The system keeps improving. The July 2022 update added keyword filtering. The September 2025 expansion added full device coverage. Next? Experts predict the FDA will start using AI to auto-suggest keywords based on your medical history or past subscriptions.
There’s also talk of expanding Early Alerts to food and supplements-areas where contamination and mislabeling are growing concerns.
For now, the best thing you can do is subscribe. It takes two minutes. And it could save your life-or someone you love’s.
Do I need to pay to subscribe to FDA Safety Communications?
No. The FDA’s safety communication subscription service is completely free. There are no hidden fees, no premium tiers, and no ads. You just need a valid email address.
Can I unsubscribe later?
Yes. Every email you receive includes an unsubscribe link at the bottom. Click it, and you’ll be removed from the list immediately. You can always resubscribe later if you change your mind.
What if I miss an alert because I didn’t use the right keyword?
The system only sends alerts based on your exact keywords. If you search for "insulin" but your device is called "Omnipod," you won’t get notified about Omnipod recalls. Always use the full product name as it appears on the label. If you’re unsure, check the FDA’s recall database directly every few months.
Are these alerts only for people in the U.S.?
The FDA’s system is designed for U.S.-based users, but anyone with an email address can subscribe. If you live outside the U.S. but use FDA-regulated products-like insulin made in the U.S. or a medical device imported from America-you’ll still benefit from these alerts. Just make sure your shipping or prescription info matches the product’s origin.
How often do these alerts come out?
It varies. Some weeks, you might get nothing. Other weeks, especially after a major incident, you might get multiple alerts in one day. In 2022, the FDA issued over 200 safety communications across all product categories. The frequency depends on your keywords and how many issues arise in those areas.
Can I get alerts for food recalls too?
Yes. The Enforcement Report Subscription Service covers all FDA-regulated products, including food. Subscribe to keywords like "peanut," "listeria," "salmonella," or "baby formula" to get food recall alerts. For meat and poultry recalls (regulated by USDA), you’ll need to sign up separately at USDA.gov.
Final Thought: Don’t Wait for a Crisis
The FDA doesn’t send alerts because it wants to scare you. It sends them because it wants to prevent harm. These systems exist because people have died from outdated devices, contaminated medications, and hidden allergens.
Subscribing isn’t a chore. It’s a shield. And the best part? You don’t need to be a doctor, a scientist, or a tech expert to use it. Just a person who cares about safety.
Set it up today. Check your email tomorrow. And if you’ve already subscribed? Tell someone you care about. Someone who might not know this service exists.




December 3, 2025 AT 18:56
Simple. Free. Life-saving.
Subscribe. Done.