Interchangeable Biosimilars: What They Are and How They Save Money
When you hear interchangeable biosimilars, a type of biologic drug approved by the FDA to be substituted for the original brand-name drug without needing a new prescription. Also known as biosimilar substitutes, these drugs are designed to work just like their expensive originals—like Humira or Enbrel—but cost far less. This isn’t just theory; it’s already changing how millions get treated for arthritis, cancer, and autoimmune diseases.
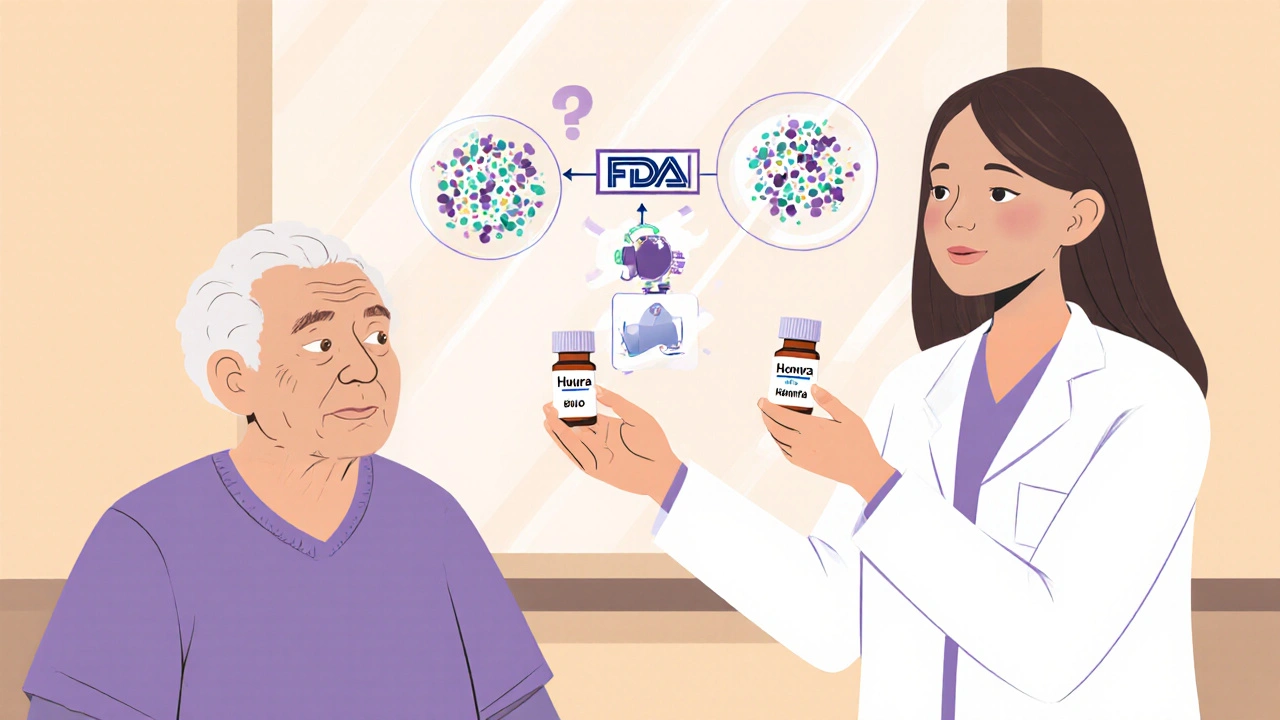
Not all biosimilars are interchangeable. A regular biosimilar is very similar to the original, but a pharmacist can’t swap it automatically unless the FDA gives it the interchangeable, a special status that means it can be substituted at the pharmacy without the prescriber’s involvement. This status requires extra testing to prove it won’t cause different safety or effectiveness results than the original. Think of it like a generic pill you can grab off the shelf—but for complex biologic drugs made from living cells, not chemicals. That’s why only a handful have earned this label so far. When a drug is labeled interchangeable, your pharmacist can switch it without calling your doctor—saving time, paperwork, and often hundreds of dollars per month.
Interchangeable biosimilars don’t mean lower quality. They’re held to the same strict standards as the original biologics. The FDA requires real-world data showing patients don’t have worse outcomes when switched. This matters because biologics are some of the most expensive drugs on the market. If you’re on a drug like adalimumab or insulin glargine, switching to an interchangeable version could cut your out-of-pocket costs by 50% or more. And it’s not just about price—many insurance plans now push for these switches to keep premiums down.
But here’s the catch: not all states let pharmacists make the switch automatically. Some require the doctor to give permission first. Others have rules about when the switch can happen. If you’re considering a switch, ask your pharmacist if the drug you’re taking has interchangeable status—and whether your state allows automatic substitution. You should also check if your insurance covers it without extra steps.
Behind the scenes, this shift is tied to how drug makers prove bioequivalence. You’ll see terms like partial AUC, a precise measurement used in testing how the body absorbs and uses a drug over time. This tool helps regulators compare how closely a biosimilar matches the original in real patients, especially when traditional metrics fall short. That’s why some of the posts below dive into bioequivalence testing, drug substitution rules, and how pharmacists ensure label accuracy when handling these complex drugs.
What you’ll find below are real, practical guides on how these drugs are tested, prescribed, and switched safely. From how to handle a substitution at the pharmacy to what happens when you switch from one biologic to another, these posts cut through the noise. Whether you’re a patient, caregiver, or pharmacy professional, you’ll get clear answers—no jargon, no fluff, just what you need to know to make smart choices about your treatment.
Pharmacists play a vital role in biosimilar adoption by counseling patients, ensuring safe substitution, and overcoming prescriber resistance. Unlike generics, biosimilars require specialized knowledge and careful communication to build trust and improve access to affordable biologic treatments.