When a patient walks into the pharmacy with a prescription for a biologic drug like Enbrel or Humira, they’re not just getting a pill. They’re getting a complex, high-cost treatment made from living cells. And if that prescription is for a biosimilar, the pharmacist’s role becomes even more critical. Unlike generic drugs, which are exact chemical copies of brand-name pills, biosimilars are highly similar-but not identical-to their reference biologics. That small difference changes everything: how they’re made, how they’re regulated, and most importantly, how pharmacists must counsel patients and handle substitution.
Why Biosimilars Aren’t Like Generics
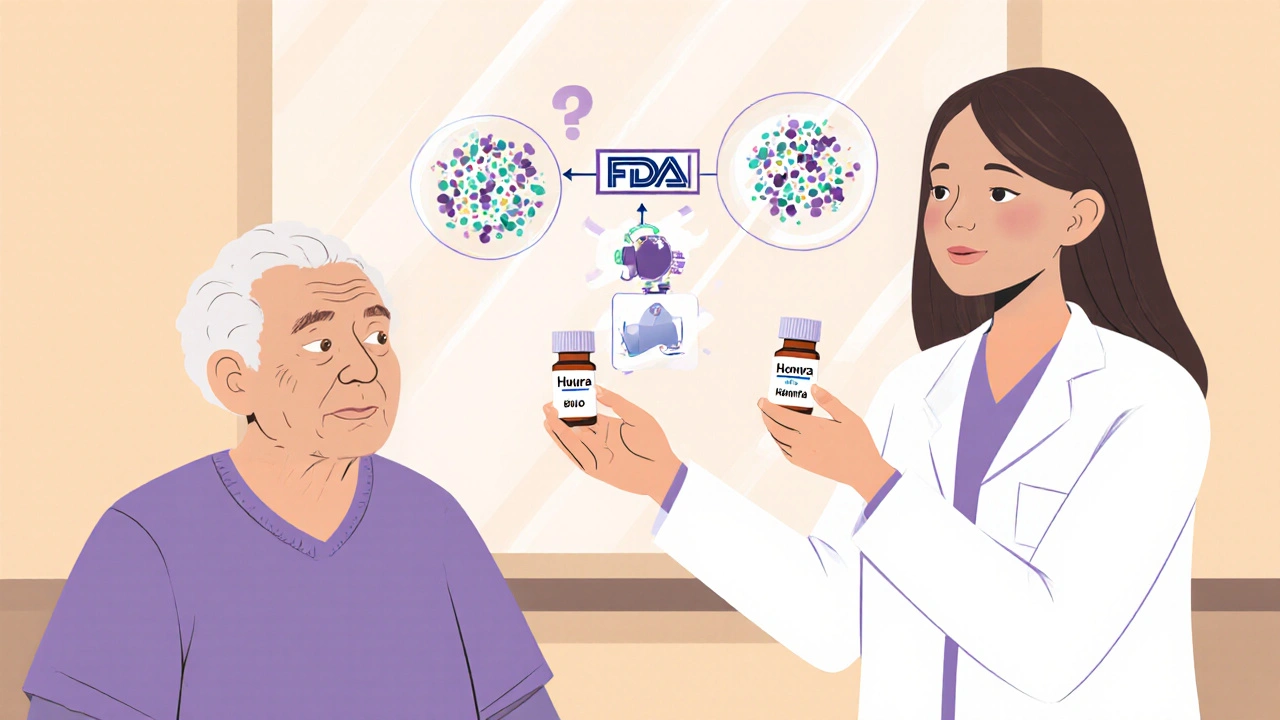
People often assume biosimilars work the same way as generic drugs. They don’t. A generic version of, say, lisinopril is chemically identical to the brand-name version. You can swap them without any concern. Biosimilars? They’re made from living organisms-cells grown in bioreactors. Even tiny changes in temperature, pH, or manufacturing conditions can lead to minor structural differences. That’s why the FDA requires extensive testing to prove biosimilars have no clinically meaningful difference in safety, purity, or potency compared to the original biologic. But they’re not exact copies. And that’s where confusion starts.That’s also why pharmacists can’t just swap them automatically like generics. Only interchangeable biosimilars-those that meet even stricter FDA standards-can be substituted without the prescriber’s permission. As of late 2023, only a handful of biosimilars have received this designation. Most still require the doctor to explicitly allow substitution. Pharmacists need to know which ones are interchangeable, which aren’t, and what state laws allow.
What Pharmacists Actually Do When Substituting
Substituting a biosimilar isn’t a simple checkbox on a computer screen. It’s a multi-step process. First, the pharmacist checks if the biosimilar is approved as interchangeable. Then, they verify state law: some states require patient notification, others require documentation of the substitution in the medical record, and a few still prohibit substitution entirely unless the prescriber says otherwise.Then comes the real work: counseling. Patients hear "biosimilar" and think "cheap copy." They worry it won’t work as well. One pharmacist at a cancer clinic shared a story: a patient with rheumatoid arthritis refused to switch from the originator drug because she thought the biosimilar was "less powerful." The pharmacist sat down with her, showed her the FDA’s data on clinical equivalence, explained that the same immune system response was measured in thousands of patients, and even pointed out that the biosimilar had been used safely in Europe for over a decade. She agreed to try it. Two months later, she came back saying she felt the same-no side effects, no flare-ups.
That’s the power of pharmacist-led education. Studies show pharmacists are far more likely than physicians to recommend biosimilars-87% versus 62%. Why? Because they spend more time with patients. They’re the ones who answer questions about cost, side effects, and safety. They’re also the ones who track whether the patient keeps taking the medication. Research shows that if the pill looks different-color, shape, size-patients are 21% more likely to stop taking it. That’s not just about confusion. It’s about trust. And pharmacists are the ones who rebuild it.
The Real Impact: Cost Savings and Provider Relief
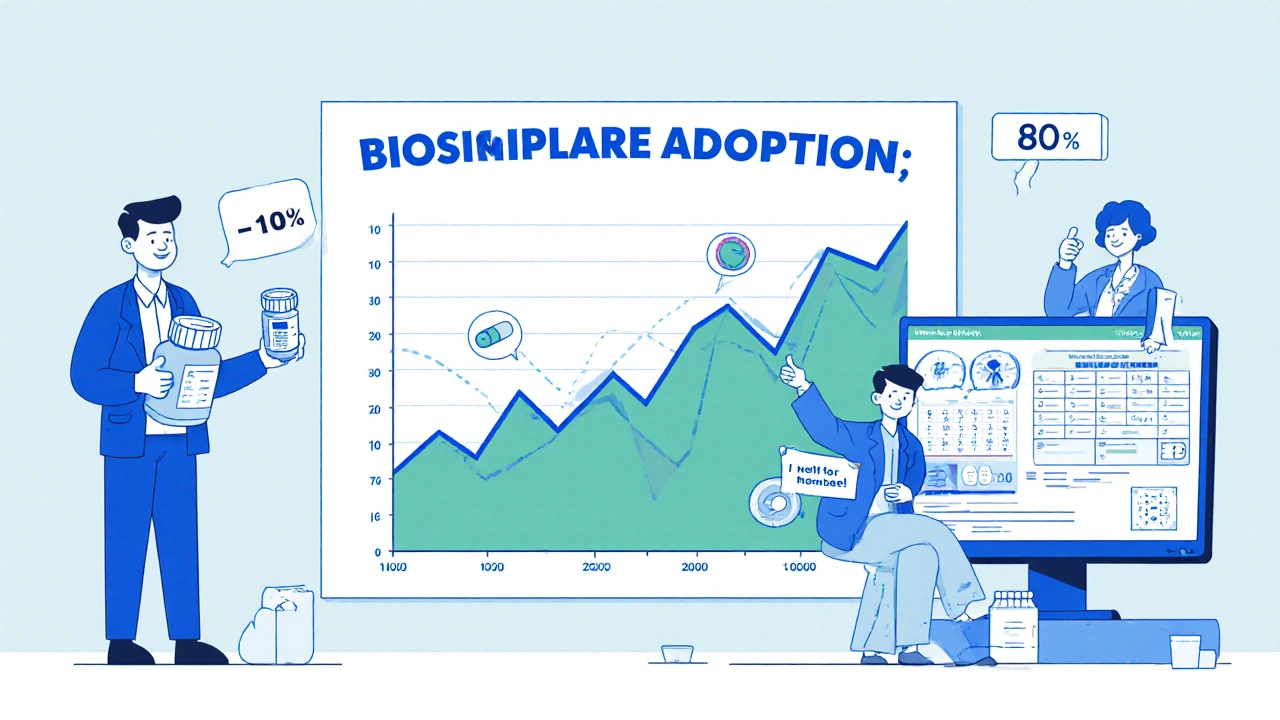
Biologics make up just 2% of all prescriptions in the U.S., but they account for nearly half of all prescription drug spending. That’s billions of dollars. Biosimilars can cut those costs by 15% to 35%, sometimes more. But savings don’t happen unless the drug gets used. And that’s where pharmacists make the difference.At the US Oncology Network, pharmacists took charge of biosimilar substitution after a major training program. Before, doctors had to manually approve each switch. That meant phone calls, delays, and frustration. After switching to a pharmacy-driven model-with providers signing off on a standard policy-substitution became automatic. Within months, adoption of biosimilars for pegfilgrastim (a drug used to boost white blood cells after chemo) jumped from under 10% to over 80%. Daily interruptions for approvals dropped to zero. Providers got back hours they’d lost to paperwork. Patients got their meds faster. And the system saved money.
That’s not an outlier. It’s a blueprint. When pharmacists are empowered to act, adoption rises. When they’re left out, it stalls.

Barriers Pharmacists Face
It’s not all smooth sailing. Many prescribers still resist. Some believe biosimilars are inferior. Others fear liability. One pharmacist on a medical forum described how a doctor yelled at them for substituting a biosimilar-then insisted on "dispense as written" for all biologics going forward. That kind of resistance isn’t rare.Then there’s the reimbursement mess. Insurance companies sometimes reward the use of the original biologic through rebates, making it cheaper for the pharmacy to dispense the more expensive drug. That’s not just bad policy-it’s bad for patients. Pharmacists are caught in the middle: they know the biosimilar is safe and cheaper, but the system pushes them toward the pricier option.
Documentation is another hurdle. Every time a biosimilar is dispensed, the batch number must be recorded. Why? Because if a patient has a reaction, regulators need to know exactly which version they got. This traceability is critical for safety. But it adds steps to the workflow. Not all electronic health records are set up to handle it easily.
What Pharmacists Need to Succeed
To make biosimilar substitution work, pharmacists need three things: training, authority, and support.- Training: 79% of pharmacists have taken continuing education on biosimilars, compared to just 43% of physicians. But not all programs are equal. The best ones cover the science, the laws, the counseling techniques, and the billing rules. Pharmacies need to invest in ongoing learning-not just a one-time webinar.
- Authority: Pharmacists need clear legal backing to substitute interchangeable biosimilars without calling the doctor every time. That means updated state laws that mirror generic substitution rules, but with added safeguards like patient notification and traceability.
- Support: Pharmacies need systems in place. Electronic alerts when a biosimilar is available. Pre-written patient education handouts. Easy ways to document substitutions in the EHR. And most importantly, a culture that trusts pharmacists to make clinical decisions.
The Future Is in Their Hands
The FDA has approved over 40 biosimilars since 2015. More are coming. The market for biologics is growing. And the pressure to lower costs is only increasing. Pharmacists are no longer just dispensers of pills. They’re clinical advisors, patient advocates, and system changers.They’re the ones who turn hesitation into acceptance. They’re the ones who catch misunderstandings before they become non-adherence. They’re the ones who keep patients on life-changing treatments by explaining, in plain language, why a biosimilar isn’t a compromise-it’s a smart, safe, and proven choice.
As one pharmacist put it on a Reddit thread: "When I explain that the FDA requires biosimilars to have the same clinical effect with no meaningful differences, most patients become comfortable with the switch." That’s not magic. That’s expertise. And it’s exactly what this system needs more of.
Can pharmacists substitute biosimilars without a doctor’s permission?
Only if the biosimilar has been designated as "interchangeable" by the FDA and state law allows it. As of late 2023, only a few biosimilars have this status. For non-interchangeable biosimilars, substitution requires the prescriber to allow it. Pharmacists must check both the FDA designation and their state’s specific laws before making any switch.
Why are biosimilars more expensive than generics?
Biosimilars are made from living cells, not synthesized chemicals like generics. The manufacturing process is complex, requiring specialized facilities and strict quality controls. Developing a biosimilar can cost hundreds of millions of dollars, compared to a few million for a generic. While they’re cheaper than the original biologic, they’re still more expensive to produce than traditional generic pills.
Do biosimilars work as well as the original biologic?
Yes. The FDA requires biosimilars to show no clinically meaningful difference in safety, purity, or potency compared to the reference product. Thousands of patients have been studied in clinical trials, and real-world data from Europe and the U.S. confirm they work just as well. Switching between the original and biosimilar doesn’t increase risk of side effects or reduce effectiveness.
Why do some patients refuse to take biosimilars?
Many patients believe "biosimilar" means "less effective" or "experimental." Others are confused when the pill looks different-color, shape, or size changes can trigger fear of a new drug. Some have had bad experiences with generics and assume biosimilars are the same. Pharmacists can overcome this by using clear language, showing FDA data, and sharing stories of other patients who switched successfully.
How do pharmacists document a biosimilar substitution?
Pharmacists must record the exact product dispensed, including the manufacturer’s name and batch number. This is required for pharmacovigilance-if a patient has a reaction, regulators need to trace which version they received. Documentation must be entered into the patient’s electronic health record and often requires patient notification, depending on state law. Failure to document properly can create safety risks and legal issues.
Are biosimilars covered by insurance the same way as the original biologic?
Sometimes, but not always. Some insurance plans still favor the originator biologic through rebate deals with manufacturers, making the biosimilar less financially attractive for pharmacies. Other plans have stepped up and incentivized biosimilar use by lowering patient copays. Pharmacists need to know their plan’s formulary rules and help patients understand their out-of-pocket costs before switching.




November 26, 2025 AT 18:21
Biosimilars are like a really good cover band 🎸-same energy, same soul, just a different stage setup. Patients need to know it’s not a downgrade, it’s a smart upgrade. And pharmacists? They’re the ones keeping the concert going without the drama.