BE Testing: What It Is, Why It Matters, and What You Need to Know
When you pick up a generic pill at the pharmacy, you might wonder: BE testing, bioequivalence testing that proves a generic drug performs the same as the brand-name version. Also known as bioequivalence study, it’s the quiet gatekeeper that makes affordable meds safe and reliable. Without it, a generic version of your blood pressure pill could be too weak—or too strong—to work right. BE testing isn’t just paperwork; it’s the science that confirms your body absorbs the same amount of medicine at the same speed, no matter the brand.
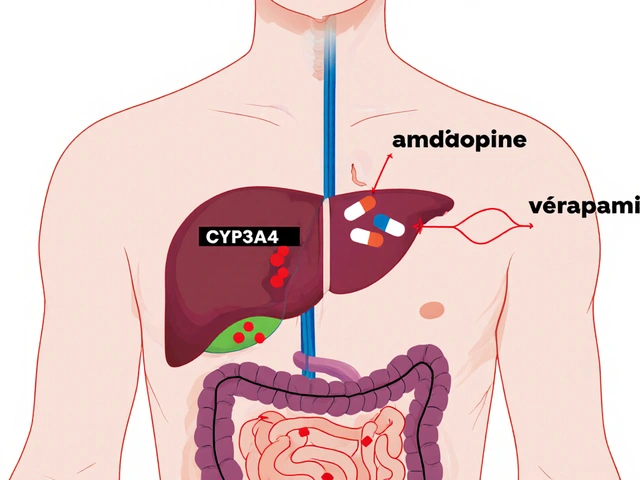
This process doesn’t just apply to any drug—it’s required for generic drugs, medications that copy the active ingredient, dose, and route of a brand-name drug to hit the market. Think of it like matching the engine in two cars: one built by the original maker, the other by a copycat. If the fuel burns differently, the car won’t run the same. BE testing checks exactly that—how fast and how much of the drug enters your bloodstream. It’s measured through blood samples taken over hours, tracking peak levels and total exposure. If the numbers fall within strict limits (usually 80–125% of the brand), the drug is approved. This isn’t guesswork. It’s regulated by agencies like the FDA and EMA, and backed by real clinical data.
BE testing also connects directly to drug absorption, how quickly and completely a medicine enters your system after taking it. A pill might have the same chemical formula, but if it dissolves too slowly or gets blocked by food, it won’t work. That’s why BE studies often test drugs under fasting and fed conditions. It’s why some generics work better with meals, and others don’t. And it’s why you can’t assume all generics are identical—even if they contain the same active ingredient. The fillers, coatings, and manufacturing methods all matter.
What does this mean for you? If you’ve switched from a brand-name drug to a generic and felt different, it’s not in your head. BE testing helps ensure consistency, but it doesn’t guarantee identical experiences for every person. Some people are more sensitive to small changes in absorption. That’s why doctors sometimes stick with brand names—for conditions like epilepsy, thyroid disorders, or blood thinners, where tiny differences can have big consequences. But for most people, BE-tested generics are just as safe and effective, and they save hundreds of dollars a year.
Behind every generic you buy, there’s a BE study. It’s not flashy. It doesn’t make headlines. But it’s the reason you can trust your prescription—even when the price tag is half what it used to be. Below, you’ll find real-world guides on medications that rely on this science: from antihistamines and sleep aids to antibiotics and blood pressure pills. These aren’t abstract concepts. They’re the drugs you take, and BE testing is what makes sure they do what they’re supposed to.
Partial AUC (pAUC) is a precise pharmacokinetic tool used to assess bioequivalence in complex drug formulations where traditional metrics like Cmax and total AUC fall short. Learn how it works, where it's required, and why it's changing generic drug approval.