Theophylline Clearance Adjuster
Select all medications being started that may interact with theophylline
Results will appear here
Take a 72-year-old man with COPD. He’s been on theophylline for years-stable, breathing okay, no issues. Then his doctor prescribes cimetidine for heartburn. Three days later, he’s in the ER with vomiting, a racing heart, and a theophylline level of 24.7 mcg/mL. Normal range? 10 to 20. He’s toxic. And it wasn’t his fault. It wasn’t even his doctor’s fault. It was a silent, well-documented interaction that slipped through the cracks.
Theophylline isn’t used much anymore. Newer inhalers, better bronchodilators, fewer side effects. But it’s still out there. Especially in older patients, in places where newer drugs cost too much, or when asthma won’t quit despite everything else. And when it’s used, it’s a tightrope walk. One wrong drug, one missed check, and you’re in danger.
Why theophylline is so easy to mess up
Theophylline doesn’t just sit in your body. It’s constantly being broken down-mostly in the liver, by an enzyme called CYP1A2. About 90% of it gets metabolized this way. The rest? Mostly peed out unchanged. That’s unusual. Most drugs are cleared through multiple paths. Theophylline? It’s all on one road. And that road is narrow.
Its therapeutic window is tiny. Too little? It won’t help your breathing. Too much? You get seizures, irregular heartbeats, even death. The FDA says over 2,000 emergency visits a year in the U.S. alone are tied to theophylline toxicity. And 35% of those? Drug interactions.
Here’s the kicker: the way theophylline is broken down doesn’t follow normal rules. At normal doses, the liver’s enzyme gets overwhelmed. That’s called non-linear pharmacokinetics. So if your clearance drops by just 20%, your blood level might jump by 50% or more. A small change in metabolism leads to a huge spike in concentration. That’s why even a little drug interaction can be deadly.
Medications that slow down theophylline clearance
Not all drugs are equal when it comes to messing with theophylline. Some barely touch it. Others? They’re like slamming the brakes on a speeding car.
Fluvoxamine is the worst offender. This antidepressant is a powerhouse CYP1A2 inhibitor. Studies show it can slash theophylline clearance by 40 to 50%. That’s not a suggestion-it’s a red alert. The European Respiratory Society says to avoid this combo entirely. If someone’s on fluvoxamine, theophylline shouldn’t even be considered.
Cimetidine is next. It’s an old-school heartburn drug, still used in some places. It cuts theophylline clearance by 25 to 30%. Real-world data shows it’s involved in nearly 29% of theophylline toxicity cases in hospitals. One patient in a 2023 study went from 15.2 mcg/mL to 24.7 mcg/mL in 72 hours after starting cimetidine. No dose change. Just the drug. That’s how fast it happens.
Allopurinol is tricky. It’s for gout. People think it’s harmless. But high doses-600 mg a day-reduce theophylline clearance by about 20%. Lower doses? Probably fine. But if someone’s on 600 mg, you’ve got to adjust the theophylline down. A 2021 study found allopurinol was the third most common culprit in toxicity cases.
Macrolide antibiotics like erythromycin and clarithromycin? They’re not the main players, but they still matter. They inhibit CYP3A4, which plays a minor role in theophylline breakdown. Still, they can knock clearance down by 15 to 25%. In older patients with weak livers? That’s enough to push them over the edge.
And then there’s furosemide. Some studies say it reduces clearance by 10-15%. Others say no effect. The evidence is mixed. But if someone’s on both and starts feeling nauseous or jittery? Check the level. Don’t assume it’s fine.
What you’re not thinking about: smoking and age
It’s not just pills. Your lifestyle matters too.
Smoking? It speeds up theophylline clearance. CYP1A2 gets turned on by cigarette smoke. Smokers need higher doses-sometimes 50% more. But what happens when someone quits? That enzyme shuts down. Clearance drops by 30 to 50% in just two weeks. If they’re still on the same dose? Toxicity risk spikes. And if they start cimetidine or fluvoxamine right after quitting? Double whammy.
Age? Older people clear theophylline slower. Their livers aren’t as sharp. Heart failure? Even worse. Clearance can drop from 1.2 mL/kg/h in a healthy smoker to 0.35 mL/kg/h in an elderly patient with heart disease. That’s more than a 70% drop. And most older adults are on multiple meds. The chance of a bad interaction? It’s not low. It’s high.
A 2021 study of 1,247 patients over 65 found that nearly 3 out of 10 were on theophylline plus a drug that reduced its clearance. Only 37% of them had their dose adjusted. That’s a ticking time bomb.

What to do when you can’t avoid the interaction
Let’s say a patient needs fluvoxamine for depression. Theophylline isn’t optional-it’s keeping them alive. What then?
You don’t just guess. You measure.
When you start or stop any interacting drug, check the theophylline level within 48 to 72 hours. That’s the rule from the American Association for Clinical Chemistry. Don’t wait for symptoms. Don’t assume. Test.
Reduce the dose upfront. For strong inhibitors like fluvoxamine, cut the theophylline dose by 50%. For cimetidine or allopurinol, drop it by 25%. The University of Michigan’s 2023 guidelines say that. And they’re not guessing-they’re using pharmacokinetic models based on real data.
Use a calculator. The University of Lausanne’s pharmacokinetic tool accounts for 12 variables: age, smoking, liver function, and yes-drug interactions. It predicts clearance with 90% accuracy. If you’re managing theophylline, you should be using it. Or at least know the numbers.
Why so many cases are missed
Why does this keep happening? Because the system isn’t built for it.
A 2023 survey of 412 pulmonologists found that 78.6% had seen a serious theophylline interaction in the past year. But 62.3% said their electronic health records didn’t warn them. No alert. No flag. Just a blank screen.
Pharmacists? They’re the last line. A pharmacist in a community pharmacy in Melbourne told a story: a patient came in with a new script for cimetidine. She checked his meds-there it was. Theophylline. She called the doctor. The doctor didn’t know. The patient didn’t know. They’d been on both for months. The pharmacist changed the prescription. Saved a hospital trip.
But not every pharmacy has that kind of time. Not every doctor remembers theophylline’s narrow window. And patients? They don’t know to ask.
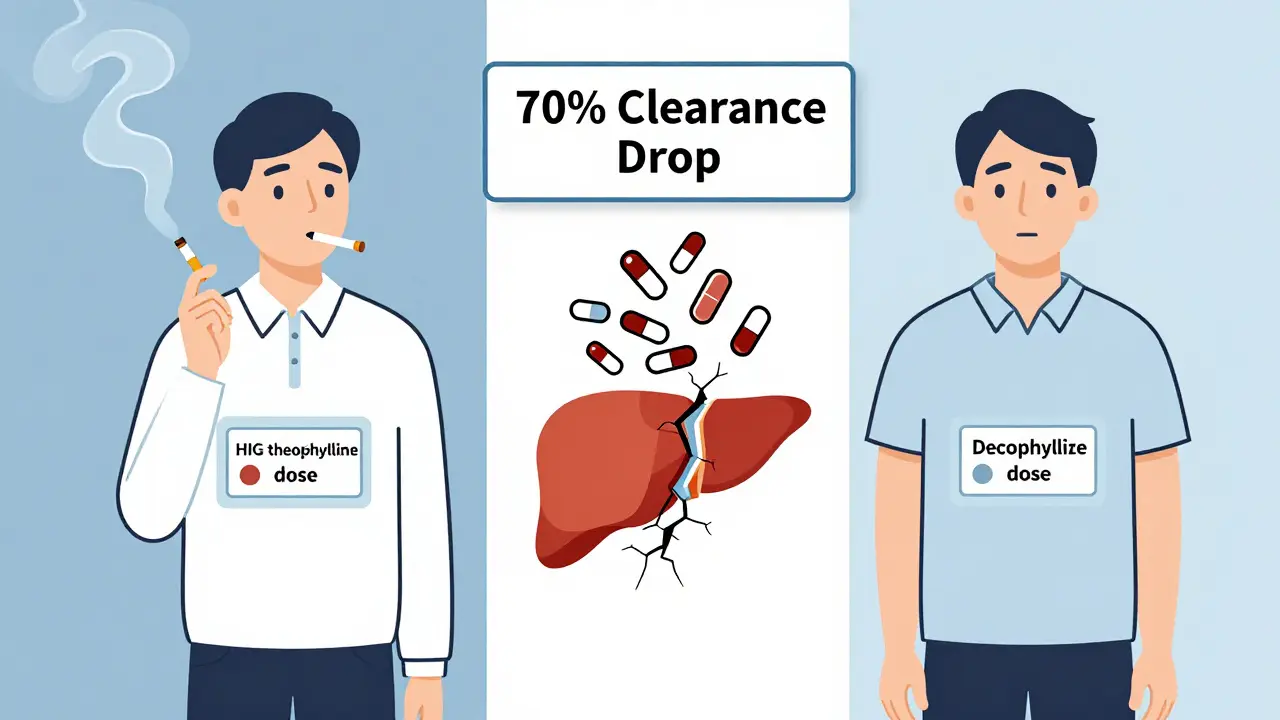
Is theophylline even worth it anymore?
Global sales have dropped 62% in the U.S. since 2000. In North America, it’s used in less than 2% of COPD cases. But in parts of Asia and Africa? It’s still 12% of treatment. Why? Because it’s cheap. Because inhalers cost too much. Because it’s all they’ve got.
And now? There’s new interest in very low-dose theophylline-for its anti-inflammatory effect in COPD, not just bronchodilation. Trials are running. But they’re excluding anyone on CYP1A2 inhibitors. They know the risk.
The European Medicines Agency says theophylline’s benefit-risk balance is still positive-if you manage the interactions. That’s the key. Not avoiding the drug. Managing the risk.
So if you’re prescribing it, you owe it to your patient to know: what else are they taking? Have they quit smoking? Are they over 65? Are they on allopurinol? Cimetidine? Fluvoxamine?
Because the difference between a stable patient and a toxic one isn’t a big dose change. It’s one forgotten pill.
Bottom line: the three rules for safe theophylline use
- Check the meds. Every time you start or stop any drug, ask: is this a CYP1A2 inhibitor? Fluvoxamine, cimetidine, allopurinol (high dose), macrolides-know them.
- Test the level. Within 72 hours of any change. Not later. Not if they feel fine. Test.
- Adjust early. Don’t wait for symptoms. If you’re adding a strong inhibitor, cut the dose by 25-50% right away. It’s safer than waiting for a seizure.
Theophylline isn’t dead. But it’s fragile. And in a world of polypharmacy, it needs more care than most drugs. The tools are there. The data is clear. The mistakes? They’re preventable.






January 4, 2026 AT 18:35
This is the kind of post that makes me question if modern medicine is just a series of lucky accidents with a side of corporate greed. We’re putting people on drugs with a margin of error thinner than a razor blade, and then acting shocked when they die? The system isn’t broken-it was designed this way. Someone’s profit margin depends on you not knowing your own meds.